Characterization of proteoforms, protein complexes and protein interactions
The analysis of intact proteins, their proteoforms, and protein complexes is a growing area of interest in the biotech industry and fundamental biology as mass spectrometers become ever higher resolving and sensitive.
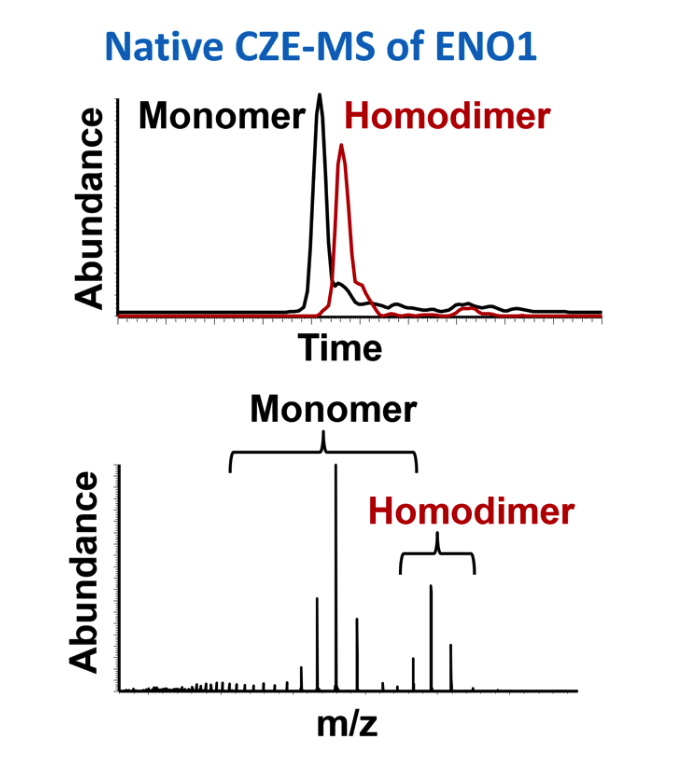
Protein biopharmaceuticals belong to an important and continually growing sector of the pharmaceutical industry. The need for high resolution separation of such challenging analytes prior to MS is essential for the characterization of biological processes, protein signaling potential therapeutic targets, and biotherapeutic proteins, especially when many of these analytes are in low abundance, isobaric or significantly decorated with diverse post-translational modifications present at various stoichiometries. Efforts are being made to develop LC columns for high resolution intact protein separation similar to the ones that we are developing for peptide separations, as mentioned above. At the same time, capillary zone electrophoresis (CZE) represents an alternative and complementary separation approach based on charge and hydrodynamic volume, with the potential for ultra-high efficiency. We and others have demonstrated the potential of high resolution CE coupled to high resolution MS to analyze intact proteins (including membrane proteins), proteoforms, protein complexes and proteomes at high sensitivity under both denaturing and non-denaturing conditions.
A. CE-MS characterization of biopharmaceutical proteins
B. CE-MS of Proteins, Protein Complexes, and Organellar Proteomes



 Belov AM, et al. Electrophoresis, 2018
Belov AM, et al. Electrophoresis, 2018